WHO’s chief guidance for containing polioviruses revised
New guidance anticipated to quicken progress in achieving necessary safeguards
Following wide engagement of stakeholders – from lab workers to engineers to certification bodies responsible for signing off on the lockdown of poliovirus strains after certification of eradication – WHO’s chief guidance document for poliovirus containment has been given an overhaul. The update to the WHO Global Action Plan for Poliovirus Containment (GAP-IV, previously GAP-III) comes at the request of the WHO Containment Advisory Group (CAG) and streamlines the tool with other relevant WHO guidance and technical recommendations made by CAG. Its availability is expected to help accelerate containment implementation worldwide.
Containment involves biosafety and biosecurity requirements for laboratories and vaccine production sites, or any other place handling and storing eradicated polioviruses, to minimize the risk of these pathogens being released into communities. It also concerns risk mitigation measures associated with field use of some live oral polio vaccines. WHO urges facilities holding virus to move through its Containment Certification Scheme, and follow guidance contained in GAP-IV.
“Retention of poliovirus materials for what their governments deem to be critical functions is a risk and responsibility for all countries that choose to do so,” said Prof David Heymann, Chair of the CAG and professor of infectious disease epidemiology at the London School of Hygiene and Tropical Medicine. “GAP provides guidance that aims to minimize the risk of escape of the poliovirus from a retention facility, and we hope that the revised publication ̶ which stakeholders in polio eradication helped shape ̶ will ensure faster action by countries that decide to retain poliovirus materials,” he said.
“The revision of the guidance has been a long time in the making and comes with a lot of anticipation,” stated Dr Harpal Singh, WHO polio technical officer and CAG secretariat. “WHO and CAG have taken on board the numerous concerns and feedback from Member States with regards to carrying out the guidance, and a certain degree of flexibility based on local risk mitigation measures has been applied in some areas, whilst maintaining the rigor of evidence-based best practice,” he added. “We anticipate that this will result in a better implementation of the requirements for Member States opting to retain [poliovirus] materials, and having their facilities certified,” he added.
To date, two of three strains of wild poliovirus have been declared globally eradicated – type 2 and type 3. Countries around the world, however, continue to handle and store these viruses for functions including polio vaccine manufacture, diagnostics and research, among others. It is essential that any facility holding poliovirus types 2 and/or wild or VDPV type 3 stocks, regardless of purpose, either put in place the necessary biorisk management measures outlined in GAP or destroy their virus stocks.
“The world is on the precipice of eradication of wild poliovirus type 1 with the lowest ever case count recorded in 2021, and we got rid of WPV2 and WPV3 in 2015 and 2019, respectively. While some progress has been made, we’re actually quite behind schedule in ensuring those two eradicated serotypes are properly contained, and more needs to be done in this regard,” said Aidan O’Leary, head of WHO’s polio eradication programme.
“Importantly, Member States all committed to accelerating poliovirus containment action in 2018 through a World Health Assembly Resolution,” he reminded. “WHO will continue to work with and encourage Member States to move on their targets, and reprioritize these actions. Failure to do so not only heightens but prolongs the risk of the reintroduction of virus, the effects of which could be devastating,” he added.
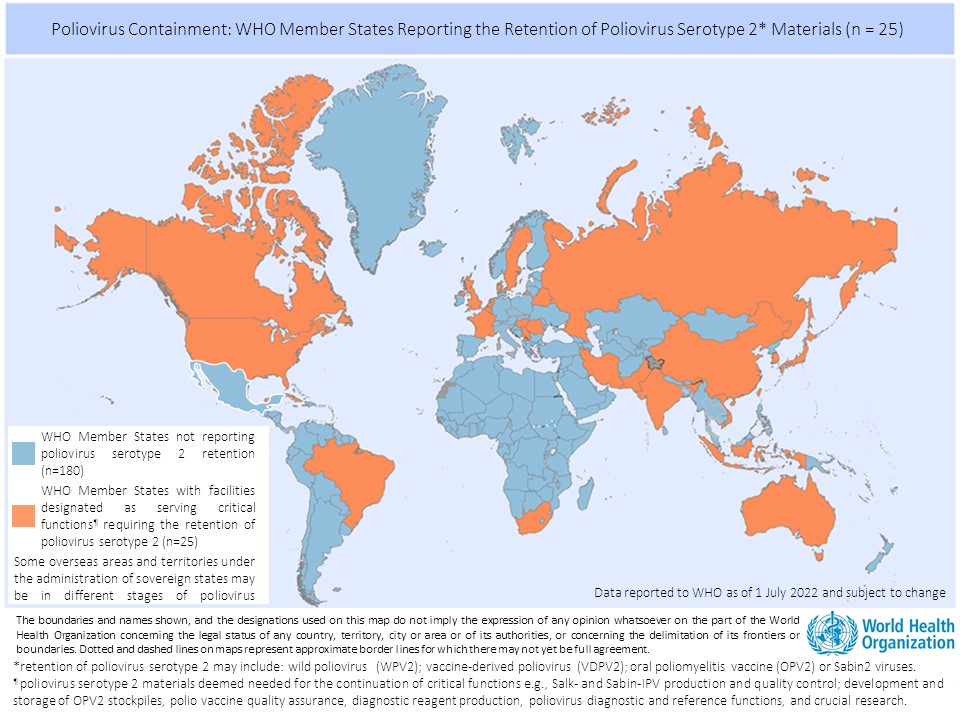
There are currently 25 countries hosting 65 facilities officially designated by their respective governments to retain poliovirus materials. The majority of countries have established a National Authority for Containment for domestic oversight over containment action, in line with commitments from the 2018 resolution, and work is underway to progress their facilities through the Containment Certification Scheme.
For now, containment measures apply to all type 2 and wild and vaccine-derived type 3 materials.